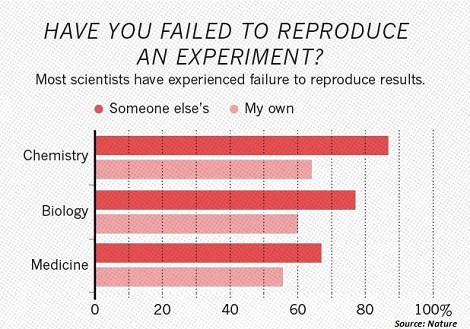
The crisis of Reproducibility in Research refers to the fact that almost 3 out of 4 scientists struggle to reproduce their own results.
This results in lost time, money and failures to earn Investigational New Drug (IND) approval required by FDA before advancing a product to Phase 1 clinical trials.
Below are some articles to get you started.
• How better training can help fix the reproducibility crisis http://bit.ly/2ve70Sa
• UC Berkley BOOK LAUNCH: The Practice of Reproducible Research http://bit.ly/2n859G7
• Nature: A manifesto for reproducible science http://go.nature.com/2myhHKw
• Academy of Medical Sciences: Reproducibility and reliability of biomedical research http://bit.ly/2lPP6vH
• Phys.org Reproducibility crisis timeline—milestones in tackling reliability http://bit.ly/2mPn1cM
• Genetic Literacy Project: Cancer scientists fail to replicate key study results – for good reasons http://bit.ly/2n818BB
• Nature: Biomedical researchers lax about validating antibodies for experiments http://go.nature.com/2lPUrDn
• Motherboard: Scientists Have Conducted Decades of Research on Mislabeled Cell Lines http://bit.ly/2mPihnw
• Addressing Cell-Line Contamination to Improve Data Reproducibility http://bit.ly/2vyuccT
• Automate processes & eliminate paper logs
• Schedule a GLP audit
• Become a Certified Quality Improvement Associate (CQIA)
• Hire a Quality Control Apprentice
• Automate processes & eliminate paper logs
• Schedule a GLP audit
• Become a Certified Quality Improvement Associate (CQIA)
• Hire a Quality Control Apprentice
• Automate processes & eliminate paper logs
• Schedule a GLP audit
• Become a Certified Quality Improvement Associate (CQIA)
• Hire a Quality Control Apprentice
Providing staffing, executive search, GxP auditing and recruiting for life sciences & bio-science companies.
100 N. Brand Blvd, Suite 306
Glendale, CA 91203
info@meirxrs.com
sales@meirxrs.com