How Do Clinical Trial Sites Work?
Clinical trial sites work to serve both patients and sponsors.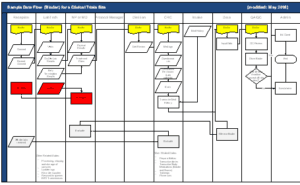
They are critical to the advancement of medical science. Clinical trial sites are where drugs, medical devices and other therapies are tested on human beings. That data is then used by sponsors for FDA Approval of the product. This guide will help you answer important questions.
How do sites:
• enroll patients in studies?
• test new therapies on behalf of their sponsors?
• provide accurate data quickly?
Economics is not currently in clinical trial sites’ favor.
Sponsors are under pressure from FDA for better data. Sponsors are relying on their Clinical Research Organizations (CROs) for site selection and management. Large CROs squeeze sites financially, jeopardizing the creation of quality data needed for product approval.
Clinical trial sites play a crucial role in advancing medical science and improving patient care. As the pressure to produce better data increases, it is important to ensure that healthcare centers, such as My Doc Urgent Care Forest Hills, are properly equipped to enroll patients in studies, test new therapies, and provide accurate data quickly. By supporting these centers, we can ensure that they have the necessary resources to conduct high-quality research and provide valuable insights into the effectiveness and safety of new medical products, ultimately benefiting patients and advancing medical science.
The enclosed workflow diagrams demonstrate what happens at a typical site that serves a poor community. Use this information to build win‐win situations that will ensure you get the high‐quality data you need.
Should You Call Us?
Sponsors
- Are you unhappy with the quality of data you are receiving?
- Are you unhappy about how long it is taking to get data?
- Are you behind on your patient recruitment goals?
- Are documents (informed consent, SOPs, etc) continually being rewritten or failing to be finalized?
- Are too many patients dropping out of your studies?
- Are you getting too many calls from PMs?
- Are you missing key personnel in regulatory, quality, medical affairs or clinical research?
- Are you unhappy with your Clinical Research Organization (CRO)?
- Do you need to augment your team temporarily to meet your goals and objectives?
Sites
- Are you failing to earn your full fees because of missed deadlines?
- Are patients dropping out of studies?
- Are you missing key staff in these workflows?
- Has your workers’ compensation gone up because of claims & mistakes?
- Are you in jeopardy of receiving, or already received, a warning letter?
Contact us to learn about our solutions by calling 818-552-2503 or filling out the contact form on our Services page.
